Les immunothérapies conçues par Transgene exploitent les mécanismes de la réponse immunitaire pour permettre au patient de se défendre contre la maladie.
Pour concevoir ses traitements, Transgene utilise un savoir-faire reconnu en ingénierie moléculaire des virus, et conçoit ainsi des produits biologiques capables d’induire une réponse immunitaire contre les cellules anormales.
Notre technologie de pointe s’appuie sur une équipe pluridisciplinaire, un savoir-faire reconnu et un large portefeuille de brevets.
Transgene dispose également d’une unité pilote de production conforme aux BPF (GMP)
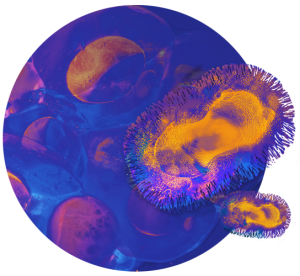
Mécanisme d’action des vaccins thérapeutiques Vaccins thérapeutiques

Les vaccins thérapeutiques détruisent indirectement les cellules malades en entraînant une cascade de réactions immunitaires, qui aboutit à la production de cellules immunitaires, appelées lymphocytes T effecteurs, capables de reconnaître et de détruire les cellules cancéreuses.
Nos produits font actuellement l’objet d’essais cliniques chez l’homme. Leur profil de sécurité, voire les premières données d’efficacité, en font des candidats médicaments prometteurs.
Deux vaccins thérapeutiques sont actuellement en clinique.
TG4050
Vaccin thérapeutique individualisé
Cancers tête et cou et cancer de l’ovaire (Phase I)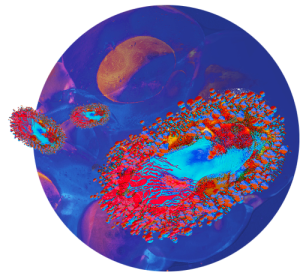
Mécanisme d’action des virus oncolytiques Virus oncolytiques

Avec les virus oncolytiques, Transgene attaque la tumeur sur plusieurs fronts.
Les oncolytiques se répliquent de manière ciblée dans les cellules malades et déclenchent une activation spécifique du système immunitaire contre ces cellules, notamment par un mécanisme de lyse cellulaire ou oncolyse.
En attaquant la tumeur avec plusieurs mécanismes d’action, Transgene développe des approches
thérapeutiques pouvant mener à une thérapie efficace contre le cancer.
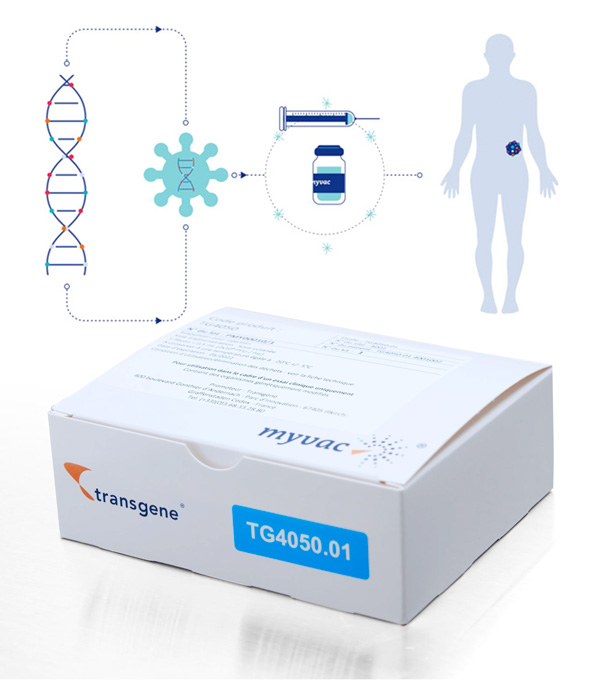
Notre approche repose sur le vecteur viral MVA déjà validé en clinique. Les produits myvac® sont conçus pour stimuler et éduquer le système immunitaire contre le cancer d’un patient en utilisant les mutations génétiques propres à sa tumeur. Une fois identifiées par séquençage et sélectionnées en utilisant des algorithmes d’intelligence artificielle, plusieurs mutations sont intégrées dans le génome du vecteur viral ; ainsi, lorsque myvac® est administré au patient, il déclenche une cascade immunitaire contre un éventail de cibles présentes dans les cellules cancéreuses. Pour créer ce traitement prometteur, Transgene a mis en place un réseau innovant couvrant bio-ingénierie, transformation numérique, génomique, approche translationnelle et notre savoir-faire reconnu en vectorisation. En mettant en commun leurs expertises, NEC et Transgene ont relevé de nombreux défis scientifiques et techniques. Transgene a aussi mis au point une unité de fabrication unique et conforme aux normes GMP, située sur son site d’Illkirch-Graffenstaden. La plateforme myvac® bénéficie du soutien de Bpifrance dans le cadre du Programme des Investissements d’Avenir. Développé en collaboration avec NEC, TG4050 est le premier candidat produit issu de myvac®. TG4050 fait actuellement l’objet d’un essai clinique de Phase I/II dans le traitement du cancer de la tête et du cou.Immunothérapie individualisée :
Un patient, un cancer, un vaccin

Avec la plateforme myvac®,
Transgene entre dans le domaine de l’immunothérapie individualisée.
myvac® en image
Cette technologie brevetée permet de générer des produits dotés d’armements multifonctionnels pour combiner plusieurs mécanismes d’action : activation du système immunitaire, destruction des cellules tumorales grâce à la lyse induite par les virus, et expression d’un armement dans la tumeur, où il pourra être également actif. Pour concevoir ces virus multifonctionnels, Transgene capitalise sur son expertise en ingénierie des vecteurs viraux et peut s’appuyer sur des collaborations. Cette technologie brevetée a déjà généré plusieurs candidats médicaments actuellement en clinique et en préclinique. Le premier virus oncolytique issu de cette plateforme technologique est armé d’un anticorps anti-CTLA-4 et de GM-CSF. BT-001 est codéveloppé avec BioInvent et est actuellement en Phase I/IIa. Fort des résultats obtenus chez les patients avec sa technologie, Transgene a lancé TG6050, un virus oncolytique exprimant l’interleukine 12 et un anticorps anti-CTLA-4. Il est administré par voie intraveineuse. Virus oncolytiques multifonctionnels :
une nouvelle génération de produits

La plateforme Invir.IO® permet de concevoir une nouvelle génération de virus oncolytiques multifonctionnels capables de moduler le micro-environnement tumoral et donc de montrer une activité antitumorale améliorée.
Publications . Pour consulter toutes nos publications, cliquez-ici